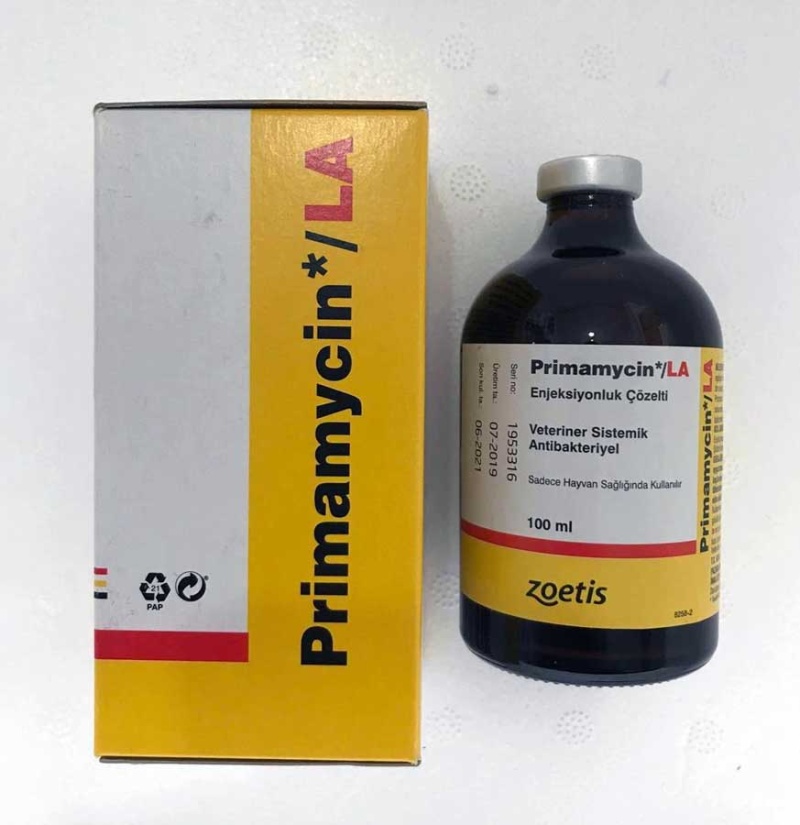
Primamycin LA - 100 ml (20%)
Primamycin LA
Primamycin LA Injectable Solution Veterinary Systemic Antibacterial Uses Only in Animal Health
Composition: Primamycin LA Injectable Solution contains oxytetracycline dihydrate equivalent to 200 mg oxytetracycline as a magnesium complex in each ml. It is a sterile, stable and concentrated solution for injection in colors ranging from yellow to amber.
PHARMACOLOGICAL PROPERTIES
Oxytetracycline is a broad-spectrum antibacterial based on the tetracycline group. Oxytetracycline inhibits protein synthesis by irreversibly binding to the 30 S subunit of the ribosome against microorganisms sensitive to it and has a bacteriostatic effect. The effects of oxytetracycline on bacteria are as follows; Gram-positive aerobes (Bacillus sp., Corynebacterium sp., Erysipelothrix rhusiopathia, Listeria monocytogenes and Streptococci.), gram-negative bacteria (Actinobacillus sp., Bordetella sp., Francisella tularensis, Haemophilus sp., Pasteurella multocida, P. haemolytica, Yersinia sp., Campylobacter fetus, Borrelia sp. and Leptospira sp., anaerobes (Actinomyces sp., Fusobacterium sp.,) and Mycoplasma sp., Chlamydia sp., Ehrlichia sp., Coxiella burnetti, Ehrlichia, Theileria, Eperythrozoon and It has a good effect on anaplasmas.
Due to acquired resistance, Staphylococci, Enterococci, Enterobacter sp., Enterobacter sp. from the Enterobacteriaceae family, E. coli, Klebsiella sp., Proteus sp., Salmonella sp., Bacteroides sp. from anaerobic bacteria. and its effect on Clostridium sp. is variable. Mycobacterium sp., Proteus vulgaris, Pseudomonas aeroginosa, Serratia sp., Mycoplasma bovis and M. hyopneumoniae are considered resistant to tetracyclines.
Oxytetracycline is excreted unchanged in urine and bile. Therefore, it is very effective in urinary system disorders. A portion of the antibiotic excreted through bile is reabsorbed from the intestines, providing enterohepatic circulation. This extends the half-life of the drug by 6-10 hours. Oxytetracycline passes through the pleura, peritoneum and meninges. In the fetal blood, it reaches a level of 1/3 of that of the mother. After parenteral administration, its concentration in bile increases to 20 times the level in blood plasma. Therefore, as a result of parenteral use, high and effective concentrations of oxytetracycline are found in the intestine. The antibiotic activity of oxytetracycline does not decrease in the presence of body fluids, serum and exudates.
Primamycin LA Solution for Injection has been specially formulated to provide long-term effective antibiotic concentration after intramuscular and subcutaneous administration. After injection, some of the drug is rapidly absorbed into the bloodstream and distributed into tissues. Peak blood concentration is reached in 4 hours. The remaining part of the drug forms a deposit at the injection site and ensures that the drug is slowly absorbed and distributed throughout the body, providing an effect that lasts for 3-5 days.
Primamycin LA AREAS OF USE and INDICATIONS
Primamycin LA Injectable Solution is indicated for the treatment of respiratory, digestive and urogenital system infections caused by microorganisms sensitive to oxytetracycline, septicemias, secondary infections that occur together with viral infections and caused by sensitive bacteria in cattle, sheep and goats. In this context, it is used as a support for local treatment in cattle and sheep, mainly in pneumonia, foot rot, septicemia, enteritis, Infectious Keratoconjunctivitis, Leptospirosis, Listeriosis, Anaplasmosis infections, as well as mastitis and metritis.
USAGE AND DOSAGE
Unless otherwise recommended by the veterinarian; Primamycin/LA Injectable Solution is administered intramuscularly and subcutaneously at a dose of 20 mg/kg body weight/day (1 ml/10 kg body weight/day). In severe infections, the second dose can be applied 3-5 days after the first application. It is recommended that the injection be made deep intramuscularly into large muscles (neck, rump). When it is necessary to use more than 20 ml for cattle and 5 ml for sheep, it is recommended to divide the total amount and inject it into two separate places. When using it, attention should be paid to asepsis and antisepsis. It should never be administered intravenously.
UNDESIRED EFFECTS / SIDE EFFECTS
Long-term use during pregnancy may cause yellow or gray discoloration in the fetus, bones and teeth. The same effect occurs in very young animals. When used long-term in high doses, it may cause fatty hepatic degeneration in pregnant animals and those with pre-existing hepatic insufficiency. Tetracyclines can cause photosensitization, allergic reaction, and a transient local reaction at injection sites. After injection, darkening of urine color may occur in cattle due to temporary hemoglobinuria.
DRUG INTERACTIONS
Tetracyclines should not be used together with beta-lactam and aminoglycoside antibiotics. They increase the nephrotoxic effect of methoxyflurane. Ampicillin sodium, amikacin sulfa